Determining the Right Beverage Panel for Compliance

As beverages venture further into the functional realm, it becomes more challenging to distinguish between beverages and supplements. Although no preapproval is required to market a product as a supplement, failing to understand the Food and Drug Administration's (FDA) definitions and distinctions can expose companies to costly legal and compliance risks.
Does Your Beverage Need a Supplement Facts Panel or a Nutrition Facts Panel?
The answer to this question is complex. Both aim to provide consumers with vital information about the product so they can make informed consumption decisions. Determining whether your beverage needs a Supplement Facts Panel or a Nutrition Facts Panel depends on how the product is formulated, marketed and intended to be consumed.
By definition, supplements and beverages are separate products, even though some functional beverages — like protein shakes and high-fiber drinks — help consumers meet their daily requirements for essential nutrients, just like a supplement does. The product's name, ingredients, packaging, serving sizes, marketing claims and label all influence whether the FDA views it as a liquid supplement or beverage.
As a result, it's crucial to familiarize yourself with the difference between a supplement label and nutrition label and their required panel components.

What Is a Nutrition Facts Panel?
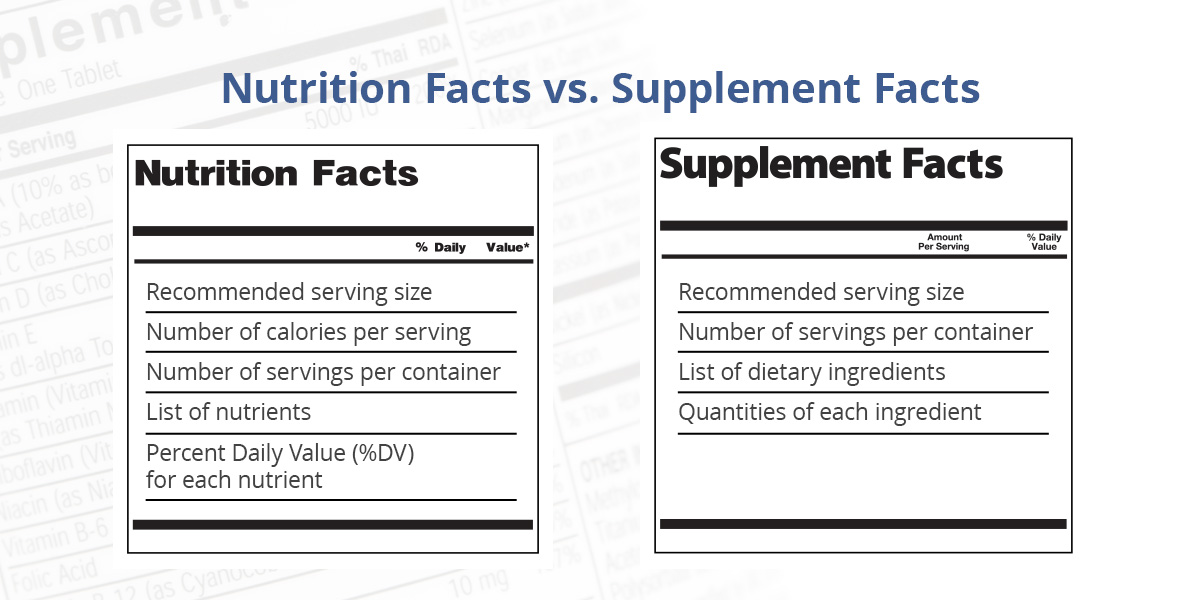
A Nutrition Facts Panel or NFP is a specific part of the product label that's used for conventional food and beverage labels. They are required by law and regulated by the FDA. The panel provides essential information about the nutritional content of the beverage and must contain product-specific information that enables consumers to make data-based choices.
What Is a Supplement Facts Panel?
A Supplement Facts Panel or SFP is used for dietary supplements, including some beverage products marketed for specific health benefits. A dietary supplement is a product that may help consumers improve or maintain their overall health, including meeting their daily requirements of essential nutrients.
Nutrition Facts vs. Supplement Facts Panels
Both must cite the recommended serving size and number of servings per container. The primary difference between a Nutrition Facts Panel and a Supplement Facts Panel is within the additional information they provide regarding their intended use.
An NFP only offers specific facts about a food product's nutritional content and is used on conventional beverages that consumers rely on for hydration, nutrition or sustenance. Conversely, SFPs are for drinks that consumers choose for a perceived health or functional benefit, rather than nutritional value.
Differences beyond this fundamental distinction include:
- Listed ingredients: An NFP lists only vitamins, minerals and nutrients. An SFP lists all ingredients with proven or purported nutritional value.
- Zero amounts: A Nutrition Facts Panel must list certain nutrients, regardless of the amounts. A Supplement Facts Panel can only list nutrients when they are present in measurable amounts — or in amounts that exceed that amount that can be qualified as "zero."
- Ingredient source: An NFP cannot list the source of a dietary ingredient, whereas an SFP can. This is typically applicable to dietary supplements derived from botanicals or other natural sources.
- Part of plant: A Nutrition Facts Panel cannot list the part of a plant from which an ingredient is derived. Conversely, a Supplement Facts Panel must include which part of the plant a dietary ingredient comes from, such as the root or leaf.
- Structure-function claims: Supplement claims can address non-nutritive and nutritive effects, while conventional beverages must focus on effects derived from nutritive value. SFPs must also include a disclaimer for any structure-function claims made.
- Proprietary blends: SFPs may disclose only the total amount of the blend, while NFPs must list each ingredient.
- Product classification: A supplement must be clearly labeled as a dietary supplement on the front label, called the Principal Display Panel.
Factors to Consider for Beverage Product Positioning
Brands should understand the important regulatory distinctions before positioning a beverage as a dietary supplement or a conventional beverage.

Ingredients
A product positioned as a beverage must contain approved food additives that are generally recognized as safe (GRAS). The FDA maintains a list of substances affirmed as GRAS in food and drinks as stated in the Federal Food, Drug and Cosmetic Act (FD&C Act). If a beverage company wants to use an ingredient that is not GRAS or falls outside the scope of GRAS regulation, the company must make a "self-affirmed" GRAS determination for the ingredient specific to the amount used and for the specific purpose intended. These reports are extensive and usually expensive to assemble.
Conversely, dietary supplement ingredients must be dietary ingredients as defined by the Dietary Supplement Health and Education Act (DSHEA). DSHEA defines dietary ingredients as:
- Vitamins and minerals
- Herbs and other botanicals
- Amino acids
- Substances used to supplement the diet by increasing total dietary intake, such as enzymes
Dietary ingredients can also include concentrates, metabolites, constituents, extracts or combinations of ingredients from these categories. Ingredients excluded from the definition of dietary ingredients — such as binders and fillers — must appear in the ingredient statement below the SFP.
The FDA isn't designed to approve dietary supplements prior to them entering the market. This places the responsibility on the brand to evaluate the safety and effectiveness of its supplements. However, the FDA can take action against any products they establish as adulterated — meaning the product is unsafe — or misbranded, such as if the labeling is misleading or false.
For example, the FDA issued a warning letter in May 2022 to Complete Nutrition for including 5-alpha-hydroxy-laxogenin as a dietary ingredient in one of their products. The FDA does not classify this ingredient as a dietary ingredient or GRAS for its intended use. Therefore, the product is an adulterated dietary supplement.
Manufacturing
Supplements have stricter manufacturing standards. Food, beverage and dietary supplement manufacturers in the U.S. follow the FDA's Current Good Manufacturing Practices (CGMPs) that govern the design, monitoring and control of manufacturing processes and facilities.
Since June 2007, the FDA has applied a different set of CGMPs to dietary supplement manufacturers. Code of Federal Regulations (CFR) Title 21 Part 111 requires certain activities to ensure dietary supplements contain what's labeled. FDA 21 CFR Part 111 also requires proof that the product doesn't include harmful or undesirable substances like pesticides or heavy metals. Additionally, the statute requires other activities, such as testing, to ensure the identity, purity, quality, strength and composition of dietary supplements.
Claims
Many regulations and guidelines surround the types of claims that are acceptable for a product. One major difference between supplements and conventional beverages comes into play when looking at structure-function claims. A structure-function claim describes the intended effect of an ingredient on the body's structure or function, such as "calcium builds strong bones" or "fiber maintains bowel regularity."
Dietary supplement products can legally make structure-function claims about their nutritive and non-nutritive effects. However, the brand must substantiate that the claim is truthful and not misleading. They must submit a notification of the claim to the FDA no later than 30 days after marketing the dietary supplement with the claim. The packaging should also contain a disclaimer that the FDA has not evaluated the claim and the product is not intended to diagnose, treat, cure or prevent any disease.
Conversely, structure-function statements for conventional beverages are limited to statements regarding taste, aroma or nutritive value. Beverage brands do not need to notify the FDA about their structure-function claims, and disclaimers are not required.
More cases are emerging in which beverage companies have allegedly overstated or misstated a product's health benefits or lack sufficient scientific substantiation for claims. Consumer class action lawyers and competitors are also suing companies for similar reasons. Unsubstantiated claims are an area that the FDA and Federal Trade Commission have addressed as a regulatory issue.
For example, in June 2024, a lawsuit emerged against Poppi for claiming that the 2 grams of prebiotic fiber in each can will benefit the gut. Consumers question whether the amount of fiber is sufficient to provide substantial health benefits. Before making any claim on your product, it's important to review and understand the rules and regulations for each of the three categories of claims — health claims, nutrient content claims and structure-function claims.
Marketing and Advertising
The area that is probably the most confusing for brands is the marketing and advertising practices that may cause a product to be deemed a beverage instead of a supplement or vice versa. Marketing includes labels, advertisements, social media or promotional activities.
The FDA issued guidance in 2014 to help beverage and dietary supplement companies determine whether they are correctly classifying their products. Some of the factors listed include serving size, recommended use, packaging and use of terms associated with beverages.
For example, product or brand names that use terms such as "beverage," "drink," "water," "juice" or similar terms could indicate that a product is a beverage and not a supplement. Additionally, promotional activities that favorably compare the product to a category of beverages or market the product based on typical beverage criteria — like taste and refreshment — signal to the FDA that the product should be classified as a beverage and not a supplement.
The FDA's main concern seems to be companies confusing consumers by switching between the two categories with one product. There is evidence that the FDA may intervene if a product with a Supplement Facts Panel and label markets or represents itself as a beverage.
In 2012, the FDA issued a warning letter to Rockstar for marketing their "Rockstar Roasted Coffee & Energy" products as supplements when they should be classified as beverages. The letter cited various factors, like the product name, packaging and statements using the word "beverage." The products also contained Ginkgo — an unapproved food additive that is not GRAS. The FDA concluded that the product was an adulterated beverage.
How to Make the Best Decision for Your Business
The overarching guidance for brands is to choose one category, know the rules and follow them. Consider your target market and business goals to aid in this decision. Where do you envision your product in a supermarket, who is your intended consumer, and what competition do you have?
Then, you can make a more informed decision about the positioning of your product and market it correctly. If you choose to make a supplement, develop, label and market a supplement. If you'd rather the product be a beverage, ensure your label and claims follow food and beverage guidelines. When in doubt, consult an experienced regulatory consultant or lawyer.

The FDA will ultimately determine whether a product is a supplement or a beverage on a case-by-case basis. Companies that understand the guidelines and build their products and strategies accordingly will mitigate the risks and costs associated with noncompliance.
Supplement and Nutrition Facts Quick Reference Guide
| Supplement Facts | Nutrition Facts | |
|---|---|---|
| Ingredients | Ingredients must be dietary ingredients as defined by the Federal Food, Drug, and Cosmetic Act | Must be GRAS and are limited to “mandatory nutrients” and a limited number of “voluntary” vitamins and minerals Required/Mandatory nutrients include total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrates, dietary fiber, total sugars, added sugars, protein, Vitamin D, calcium, iron and potassium. |
| Sources of Dietary Ingredients | May list the source of a dietary ingredient Example Hydrolyzed collagen (from bovine hide) Not required to list source of dietary ingredient if it is listed in the Supplement Facts Panel | Cannot list the source of a dietary ingredient Example: Collagen |
| Part of Plant | Must include the part of the plant the dietary ingredient is derived from by common or usual name. Listings of botanicals must specify the part of the plant from which the ingredient is derived and include Latin name. Example: Organic Ashwagandha Extract (Withania Somnifera) (roots and leaves) | Cannot list part of the plant Example: Ashwagandha |
| Zero Amounts | Cannot list "zero" amounts of any nutrients. Must only show ingredients that are present in measurable amounts. | Must list “zero“ amounts of mandatory nutrients |
| Structure Function Claims | May focus on effects derived from non-nutritive and nutritive effects Example: Calcium builds strong bones Must submit FDA notification Must include disclaimer | Must only focus on effects derived from nutritive value FDA notification is not required Disclaimers are not required |
| Proprietary Blends | May list only the total amount of the blend | Must list each ingredient |
| Front Label | Dietary Supplement | Drink, Beverage, Water, etc. |
Get Started on Your Beverage Idea
Whether you want to develop a supplement beverage or a conventional one, BevSource can help. We provide a full spectrum of services and sourcing capabilities to help you develop, produce and deliver your beverage vision from the first formula to full trucks of product. Our team has worked with beverages across various categories, from flavored water and sports drinks to smoothies and juices.
When you partner with us to bring your beverage to life, you gain access to our wealth of experience and expertise. We know what it takes to make a successful beverage and understand the challenges that come along the way. We simplify the process so you can save time and focus on your business.
When you collaborate with us, you receive more than advice. We customize our services to each client and build a custom strategy that works for your unique business. With our unmatched network of suppliers and vendors, we can identify the right services to support and execute your beverage plan.
Let's make your idea a reality together. Contact us today to speak with a beverage specialist about your product or business. We can also answer any questions you have about labeling and positioning your functional beverage.

Editor's Note: This post was originally published in September 2017. It was updated in August 2022 with new examples, insights and more accurate information.
Disclaimer: The information in this article is intended to convey general information regarding beverage regulations and compliance. It does not constitute legal advice. This is for informational purposes only, and we strongly encourage you to seek independent legal counsel for advice on specific legal issues.
